1) lewis representation of butanol lewis representation of water :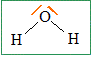
2) M(h2O) = 18g/mol
n(h2O) = m(h2O) / M(h2O)
n(h2O)=1000/18= 55.55 mol
M(C4H10O)= 74
n=m/M
=2000/74
=27 MOL
In 1kg of butanol, there are 55.5 mol
In 2kg of water, there are 27 mol.
3) equation :C3H8+ …O2–> ….CO2+….H2O
the equation complete is : C3H8+ 5O2–>3CO2+4H2O


Recent Comments